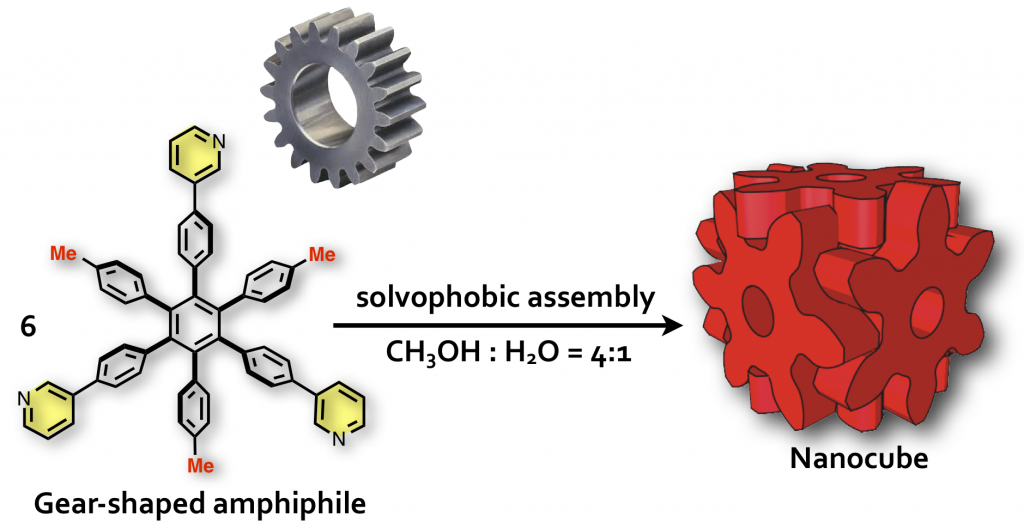
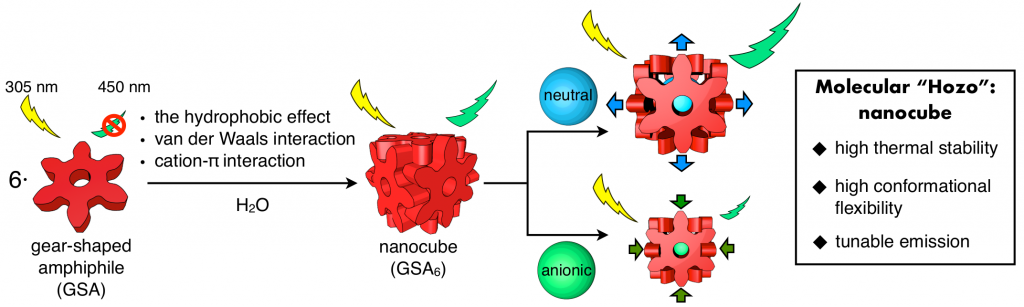
Molecular Mortice and Tenon (Molecular “Hozo”): Can we form uniquely stable self-assemblies using only weak and less directional Intermolecular Interactions?
The formation of ordered molecular self-assemblies depends on how information about the directionality of intermolecular interactions is incorporated into the building blocks. Some intermolecular interactions, such as van der Waals (vdW) force, have poor directionality and weak attractive force. However, there are examples where vdW force is used efficiently in Nature. In our laboratory, we are trying to form unique molecular self-assemblies with great stability by using weak molecular interactions that lack the direction of chemical bond such as vdW force and the hydrophobic effect. To compensate for the lack of directionality in chemical bonding, we designed an indented molecular surface on the molecules, and we have developed discrete molecular self-assemblies (nanocube) by meshing gear-shaped molecules. This idea is similar to the method known as “mortise and tenon” that engages wood without using nails or adhesives, and is named “molecular hozo” in Japanese. The use of a molecular tenon and tenon has shown that a stable molecular self-assembly can be formed in water even at 150 °C or higher, even though only weak interactions such as the vdW force and cation-π interaction are used. The goal in this research is to establish the principle of designing a molecular mortise and tenon so that a variety types of discrete molecular self-assemblies can be formed by this method.
Development of Dissipative Molecular Self-assembly Systems
The phenomenon of molecular self-assembly is essentially under chemical equilibrium, and the state where self-assembly is completed is static. Although molecular self-assembly plays an important role in life systems, molecular self-assemblies themselves are not life systems. Life activity is maintained by taking in energy from the outside and consuming it, creating an energetically unstable state and always trying to escape the equilibrium state. In this way, the metastable self-assembled state is maintained, and the dynamic self-assembled system (dissipated self-assembled system) in which the assembly and disassembly are repeated is relevant to the life system. By artificially constructing such a system, it is thought that understanding of biological systems will be deepened at the molecular level, and it will function as a molecular machine. Recently, we have used thermal energy to transiently create a state away from chemical equilibrium and build a chemical system that enables to interconvert between ordered and disordered states. In the future, we are trying to build more complicated chemical systems using other energy sources.

101. Molecular “Hozo”: Thermally Stable yet Conformationally Flexible Self-assemblies Driven by Tight Molecular Meshing
Y.-Y. Zhan and S. Hiraoka*
Bull. Chem. Soc. Jpn. in press. [DOI: doi:10.1246/bcsj.20210228] (Masterpiece Materials with Functional Excellence) (Account)
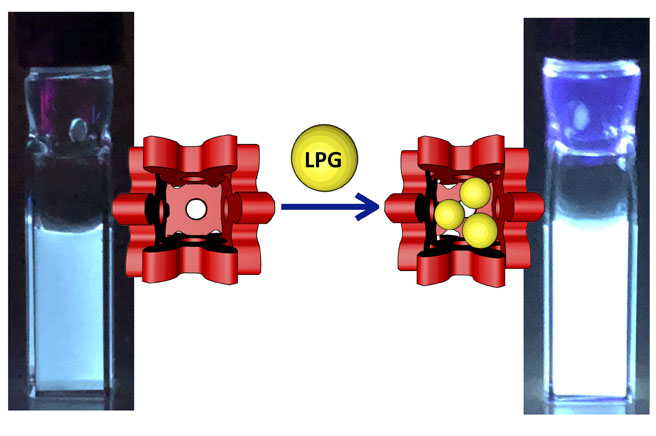
94. Supramolecular Fluorescence Sensor for Liquefied Petroleum Gas
Y.-Y. Zhan, J. Liao, M. Kajita, T. Kojima, S. Takahashi, T. Takaya, K. Iwata, and S. Hiraoka*
Commun. Chem. 2, 107 (2019). [DOI: 10.1038/s42004-019-0212-6]
Press release [Link]

Gakushuin University
93. Polarizability and Isotope Effects on Dispersion Interactions in Water
Y.-Y. Zhan, Q.-C. Jiang, K. Ishii, T. Koide, O. Kobayashi, T. Kojima, S. Takahashi, M. Tachikawa, S. Uchiyama, and S. Hiraoka*
Commun. Chem. 2, 141, (2019). [DOI: 10.1038/s42004-019-0242-0]
Press release [Link]

Yokohama City University

Osaka University
92. Molecular Dynamics Study on Dynamical Features of Reorganization Process for Nanocapsule Formed with Gear-shaped Amphiphile Molecules.
T. Mashiko, S. Hiraoka, U. Nagashima, and M. Tachikawa*
J. Phys. Chem. B in press. [DOI: 10.1021/acs.jpcb.9b02156]
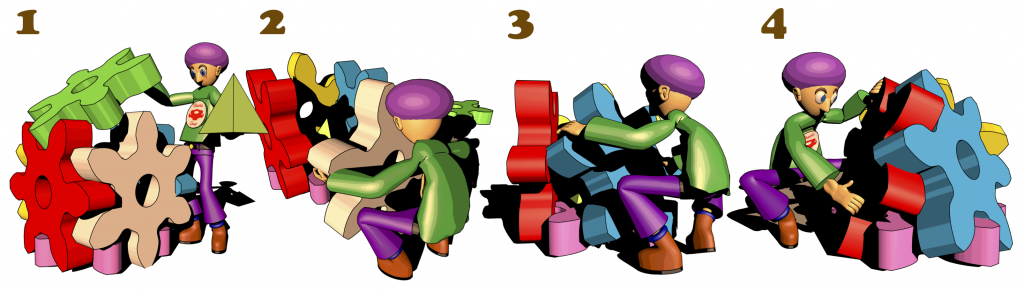

Yokohama City University
89. Temperature-Controlled Repeatable Scrambling and Self-Sorting of Building Blocks Between Cubic Assemblies.
Y.-Y. Zhan, T. Kojima, K. Ishii, S. Takahashi, Y. Haketa, H. Maeda, S. Uchiyama, and S. Hiraoka*
Nature Commun. 10, 1440 (2019). [DOI: 10.1038/s41467-019-09495-1]
Press release [Link]
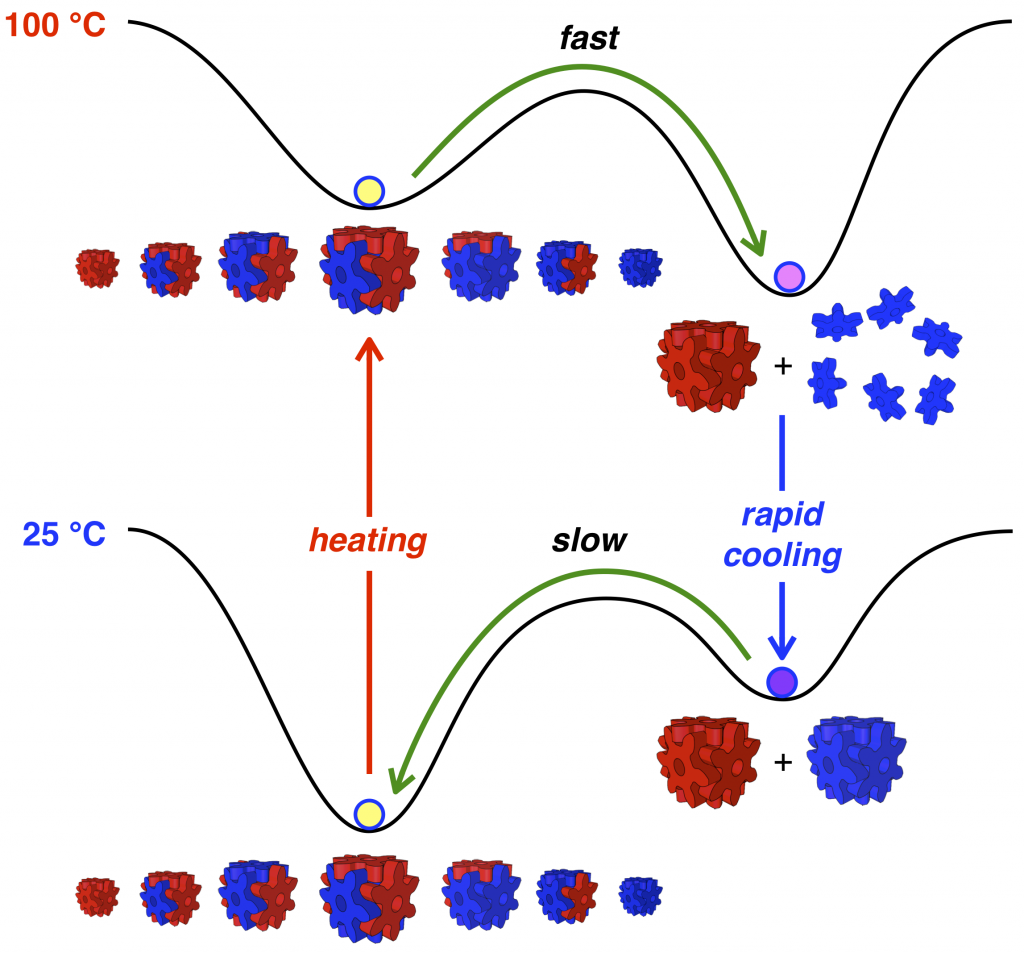

Ritsumeikan University

Osaka University
83. Self-Assembly of Nanocubic Molecular Capsules via Solvent-Guided Formation of Rectangular Blocks.
T. Yamamoto*, H. Arefi, S. Shanker, H. Sato*, and S. Hiraoka*
J. Phys. Chem. Lett. 9, 6082–6088 (2018). [DOI: 10.1021/acs.jpclett.8b02624]
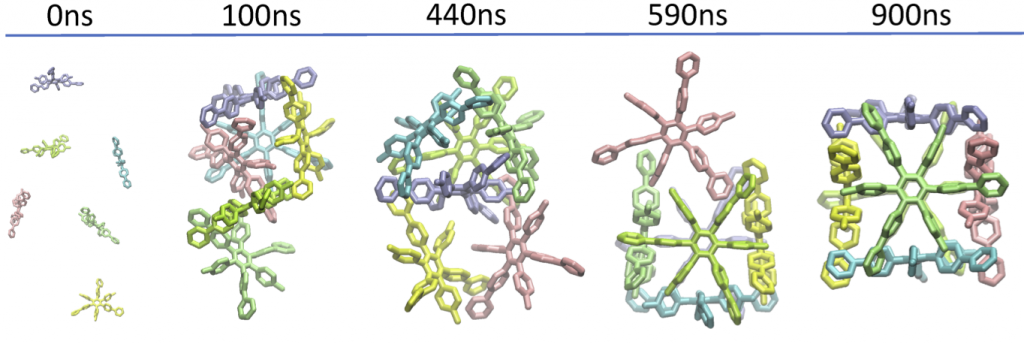

Kyoto University

Kyoto University
82. Induced-fit Expansion and Contraction of a Self-assembled Nanocube Finely Responding to Neutral and Anionic Guests.
Y.-Y. Zhan, T. Kojima, T. Nakamura, T. Takahashi, S. Takahashi, Y. Haketa, Y. Shoji, H. Maeda, T. Fukushima, and S. Hiraoka*
Nature Commun. 9, 4530 (2018). [DOI: 10.1038/s41467-018-06874-y]
Press release [Link]
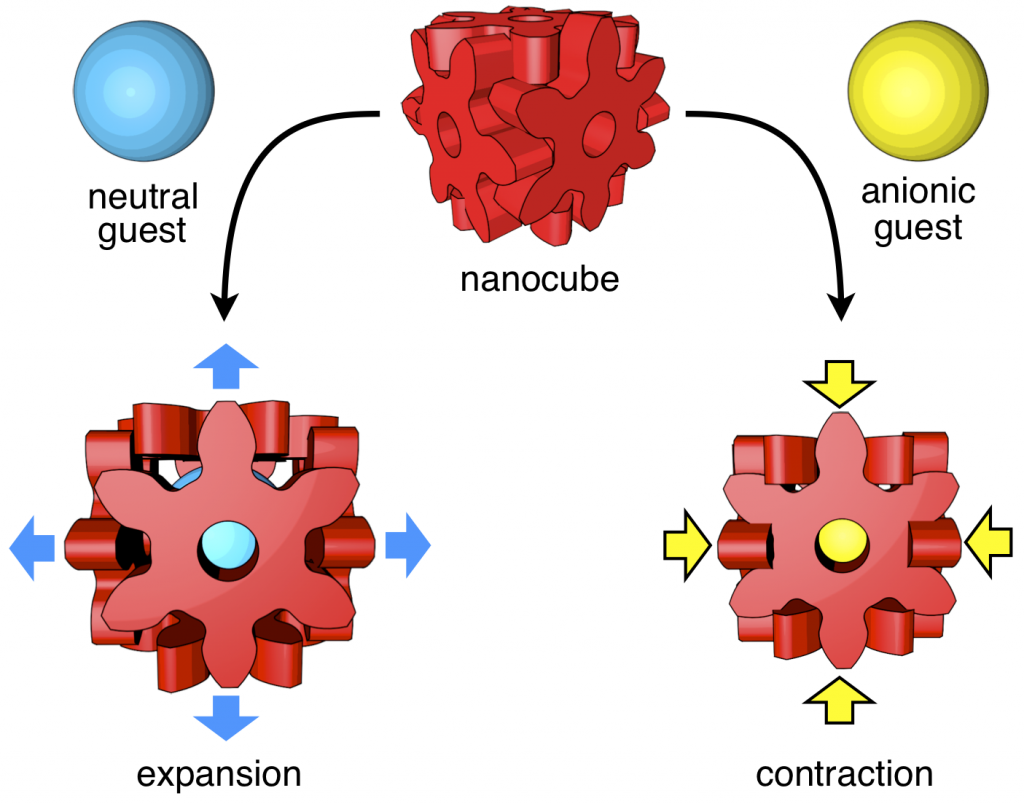

Ritsumeikan University

Tokyo Institute of Technology
van der Waals相互作用を活用した物質合成
小島 達央・平岡 秀一
化学, 73, (9), 68–69 (2018).
81. Gram-Scale Synthesis of a C2v-Symmetric Hexaphenylbenzene with Three Different Types of Substituents.
J. Liao, T. Kojima, S. Takahashi, and S. Hiraoka*
Asian J. Org. Chem. 7, 2057 – 2060 (2018). [DOI: 10.1002/ajoc.201800448] selected as Cover Feature

76. A Balance Between van der Waals and Cation-π Interactions That Stabilizes Hydrophobic Assemblies.
Y.-Y. Zhan, T. Kojima, T. Koide, M. Tachikawa, and S. Hiraoka*
Chem. Eur. J. 24, 9130 – 9135 (2018). [DOI:10.1002/chem.201801376]
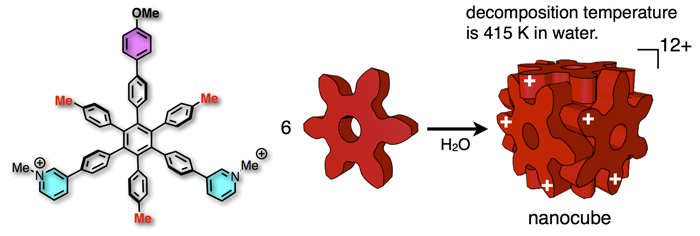

Yokohama City University
75. Importance of Molecular Meshing for the Stabilization of Solvophobic Assemblies.
Y.-Y. Zhan, N. Tanaka, Y. Ozawa, T. Kojima, T. Mashiko, U. Nagashima, M. Tachikawa, and S. Hiraoka*
J. Org. Chem. 83, 5132 – 5137(2018). [DOI: 10.1021/acs.joc.8b00495]
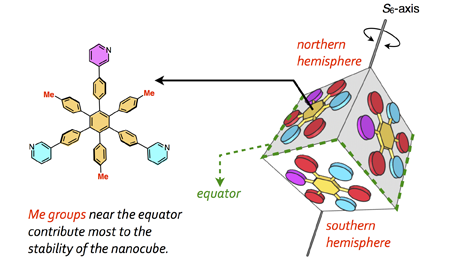

Yokohama City University
73. Unresolved Issues that Remain in Molecular Self-Assembly.
S. Hiraoka*
Bull. Chem. Soc. Jpn. 91, 957–978 (2018). [DOI: 10.1246/bcsj.20180008] Commemorative Accounts: Self-Organization

70. Programed Dynamical Ordering in the Self-organization Processes of a Nanocube: A Molecular Dynamics Study.
R. Harada,* T. Mashiko, M. Tachikawa, S. Hiraoka, and Y. Shigeta*
Phys. Chem. Chem. Phys. 20, 9115–9122 (2018). [DOI: 10.1039/C8CP00284C]
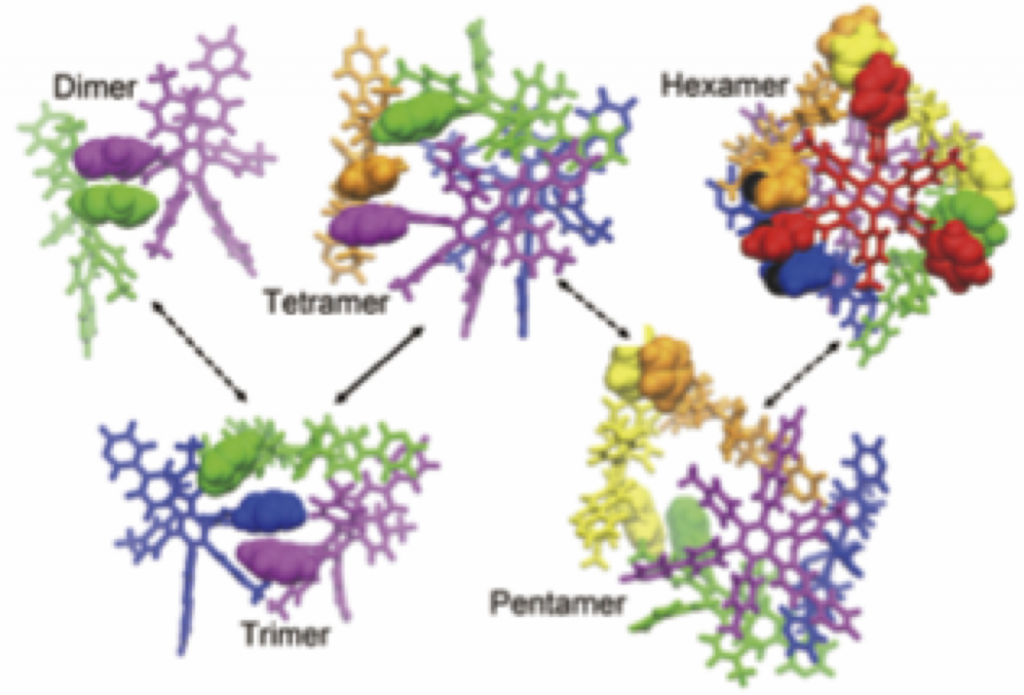

Tsukuba University

Yokohama City University
69. Semi-quantitative Evaluation of Molecular Meshing by Surface Analysis with Varying Probe Radii.
N. Tanaka, Y.-Y. Zhan, Y. Ozawa, T. Kojima, T. Koide, T. Mashiko, U. Nagashima, M. Tachikawa, and S. Hiraoka*
Chem. Commun. 54, 3335–3338 (2018). [DOI: 10.1039/c8cc00695d] selected as Back Cover

67. Hyperthermostable Cube-shaped Assembly in Water.
Y.-Y. Zhan, K. Ogata, T. Kojima, T. Koide, K. Ishii, T. Mashiko, M. Tachikawa, S. Uchiyama, and S. Hiraoka*
Commun. Chem. 1, 14 (2018). [DOI: 10.1038/s42004-018-0014-2]
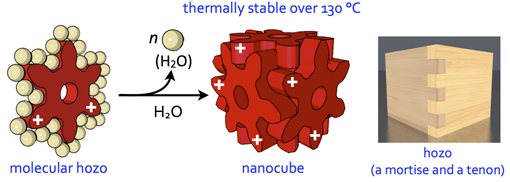

Yokohama City University

Osaka University
57. Theoretical Study on Substituent and Solvent Effects for Nanocubes Formed with Gear-shaped Amphiphile Molecules.
T. Mashiko, S. Hiraoka, U. Nagashima, and M. Tachikawa*
Phys. Chem. Chem. Phys. 19, 1627–1631 (2017). [DOI: 10.1039/c6cp07754d]

Yokohama City University
53. Molecular Dynamics Simulations of Self-Assembled Nanocubes in Methanol.
T. Mashiko, K. Yamada, S. Hiraoka, U. Nagashima, and M. Tachikawa*
Molecular Simulation 41, 845–849 (2015) [DOI: 10.1080/08927022.2014.940523]
49. Molecular Dynamics and Principal Components Analysis for a Self-assembled Nanocube in Aqueous Solution
T. Mashiko, K. Yamada, T. Kojima, S. Hiraoka, U. Nagashima, and M. Tachikawa*
Chem. Lett. 43, 366–368 (2014). [DOI: 10.1246/cl.130928]

Yokohama City University
48. Temperature Dependence of Self-Assembled Molecular Capsules Consisting of Gear-Shaped Amphiphile Molecules with Molecular Dynamics Simulations.
J. Koseki, Y. Kita, S. Hiraoka, U. Nagashima, and M. Tachikawa*
Int. J. Quant. Chem. 113, 397 (2013). [DOI: 10.1002/qua.24108]

Yokohama City University
46. Role of CH-π Interaction Energy in Self-Assembled Gear-Shaped Amphiphile Molecules: Correlated ab initio Molecular Orbital and Density Functional Theory Study.
J.Koseki, Y.Kita, S. Hiraoka, U.Nagashima, and M.Tachikawa*
Theor. Chem. Acc. 130, 1055-1059 (2011). [DOI: 10.1007/s00214-011-1053-2]

Yokohama City University
一義集合体を形成するための疎水表面エンジニアリング
平岡秀
有機合成化学協会誌,69, 671–679 (2011).
45. In-water Truly Monodisperse Aggregation of Gear-Shaped Amphiphiles Based on Hydrophobic Surface Engineering.
S. Hiraoka,* T. Nakamura, M. Shiro, and M. Shionoya*
J. Am. Chem. Soc. 132, 13223-13225 (2010). [DOI: 10.1021/ja1069135]
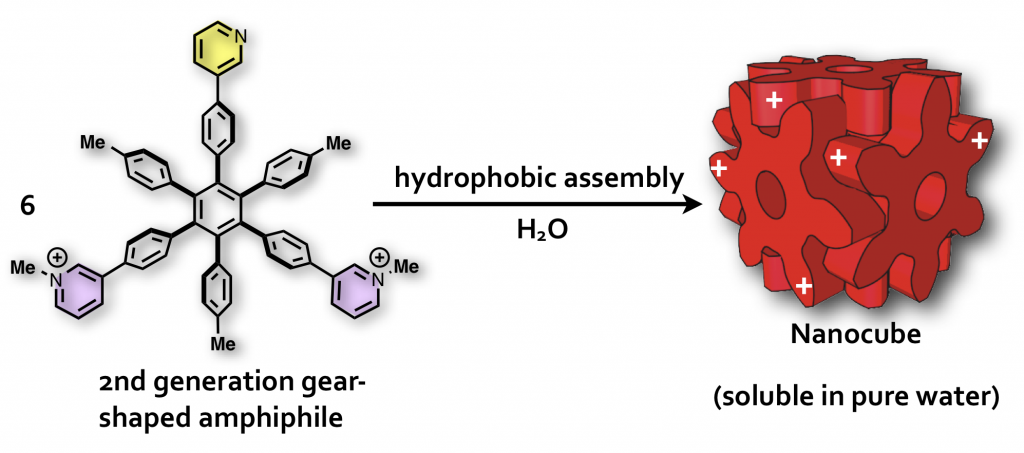
40. Induced-Fit Formation of a Tetrameric Organic Capsule consisting of Hexagram-Shaped Amphiphile.
S. Hiraoka,* K. Harano, T. Nakamura, M. Shiro, and M. Shionoya*
Angew. Chem. Int. Ed. 48, 7006-7009, (2009). [DOI: 10.1002/anie.200902652]
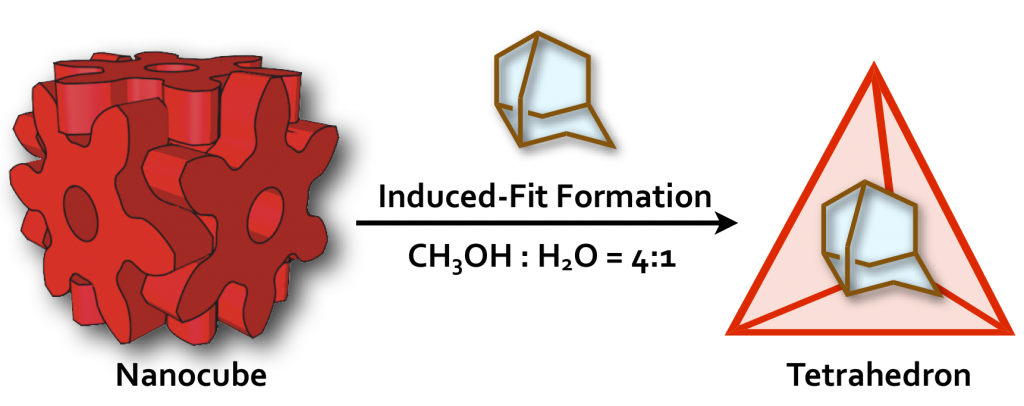
37. A Self-Assembled Organic Capsule Formed from the Union of Six Hexagram-Shaped Amphiphile Molecules.
S. Hiraoka,* K. Harano, M. Shiro, and M. Shionoya*
J. Am. Chem. Soc. 130, 14368-14369 (2008). [DOI: 10.1021/ja804885k ]